Trendaavat aiheet
#
Bonk Eco continues to show strength amid $USELESS rally
#
Pump.fun to raise $1B token sale, traders speculating on airdrop
#
Boop.Fun leading the way with a new launchpad on Solana.
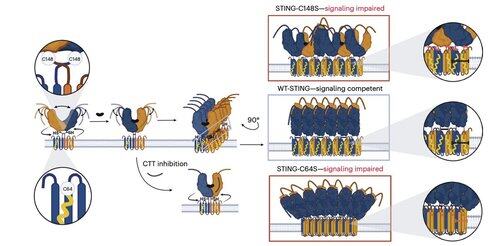
Core Investigator @li_lingyin's new paper in @NatChemBio investigates why human STING inhibitors have shown limited efficacy, despite strong activity in mouse models.
The study, led by @xujun_cao and @rjchan426, finds that the commonly targeted site on STING is not required for human signaling, and identify an alternative regulatory mechanism.

Link to paper:
Li and her colleagues show that C91 palmitoylation, the target of several inhibitor compounds including H-151, is dispensable for human STING signaling.
This helps explain why some inhibitors block STING in mouse cells but not in human immune cells.
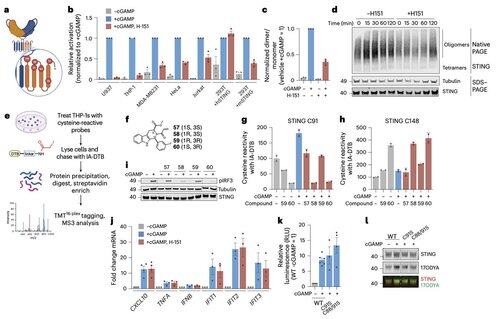
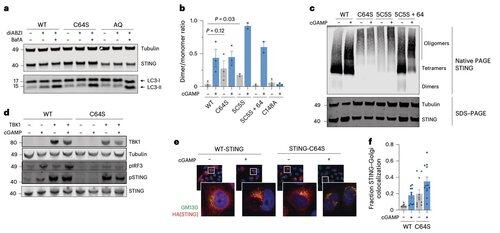
Instead, Li's team finds that C64, a conserved and basally palmitoylated cysteine, is essential for human STING activation.
C64 prevents premature assembly of STING into non-productive oligomers and serves as a key checkpoint before activation.
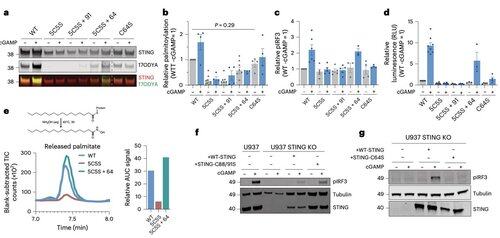
Palmitoylation at C64 and C91 regulates disulfide bonding at C148, which stabilizes STING oligomers.
This disulfide formation is necessary, but if it occurs too early, it locks STING into an inactive state.
To move beyond the limitations of context-dependent modifications, Li's team focused on the oligomerization interface itself.
This led to the discovery of a minimal 8–amino acid peptide that binds a defined, surface-exposed pocket and blocks STING activation.
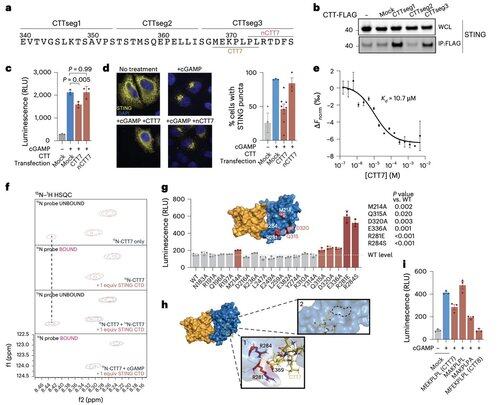
This interface defines a previously unrecognized, drug-accessible site on human STING and offers a promising direction for designing inhibitors that are effective across contexts. This sets the stage for future therapeutic development in autoimmunity and inflammation.
Learn more:
6,24K
Johtavat
Rankkaus
Suosikit













